Properties:
- 1-tert-Butyl-3-ethylcarbodiimide
- CAS 1433-27-8
- C7H14N2
- MW 126.20

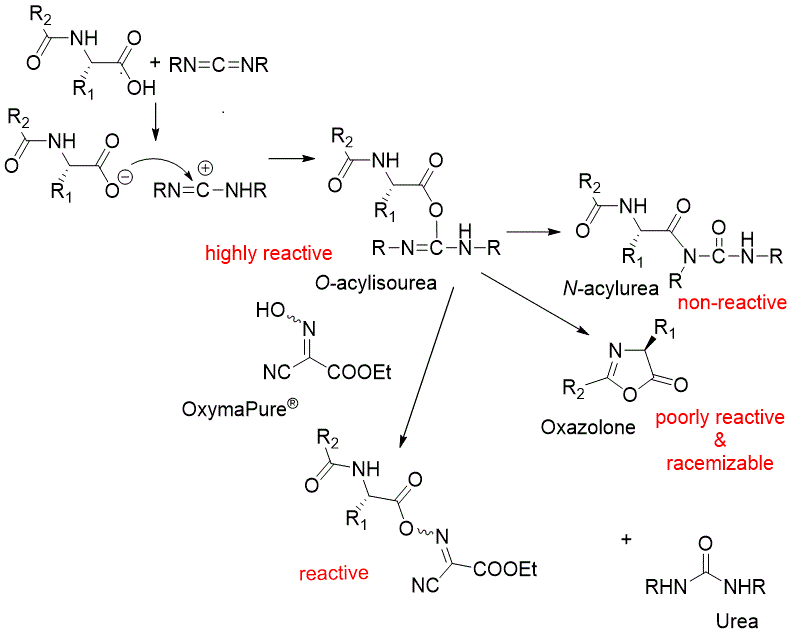
The most common strategy for the synthesis of amide bonds is the condensation of amines with carboxylic acids in the presence of carbodiimides, (EDC HCl, DIC, T-BEC). Carbodiimides react with the carboxylic group to render the highly reactive O-acylisourea (see scheme). Due to its high reactivity, O-acylisourea decomposes rapidly and/or undergoes rearrangement to form the totally inactive N-acylurea or in the case of the carbamate protected α-amino acids, it evolves to oxazolone, which shows poor reactivity and provokes the loss of chiral integrity. To mitigate this inefficiency in the carbodiimide-mediated coupling, a coupling additive such as OxymaPure®, HOBt, HOPO, or others can be added to the coupling cocktail. Since the active species formed shows increased stability, has excellent acylation capabilities, and significantly minimizes the side-reactions associated with O-acylisourea.
T-Bec®, 1-tert-Butyl-3-ethylcarbodiimide an advanced carbodiimide used with additive coupling methodology,
for peptide/amide bond formation and it is the registered trademark of Luxembourg Bio Technologies Ltd.